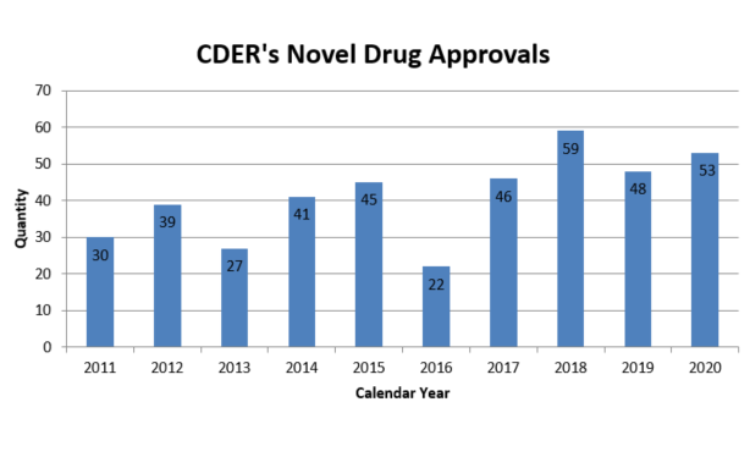
Jan. 11, 2021 – When considering all of the COVID-19 challenges the FDA faced in 2020, it is noteworthy that the Center for Drug Evaluation and Research (CDER) approved 53 novel drugs last year, the second highest number of novel drug approvals in the past 10 years.
In a forward to CDER’s 2020 report on the new drug therapy approvals, CDER Acting Director Patrizia Cavazzoni, M.D., stated that despite the hardships presented by COVID-19, “we have approved many new therapies that will advance health for the American public. This includes the first FDA-approved drug for the treatment of COVID-19.”
From 2011 through 2019, CDER has averaged roughly 40 novel drug approvals per year, with the peak being 59 approvals in 2018, followed by 48 approvals in 2019, the CDER report states.
“Last January, many predicted the FDA would approve more than 50 new drugs in 2020 – and, even with an increased workload and political attacks related to the COVID-19 pandemic, the agency did manage to accomplish this,” said Coalition for Healthcare Communication Executive Director Jon Bigelow. “That is a testament to the hard work and diligence of the CDER and CBER teams, as well as to the efficient processes that have been introduced in recent years.”
At a Nov. 19, 2020, Coalition for Healthcare Communication webinar, “Healthcare Policy in a Post-Trump World,” Prevision Policy Senior Editor Kate Rawson noted that “Commissioner [Stephen] Hahn and other top leaders at the FDA have really encouraged reviewers to keep their heads down and do the work that’s in front of them, and I think they’ve really been successful at doing that.”
Rawson also told webinar attendees that staff morale, even under the current stressful circumstances, is quite high. “While staff know that they are overworked, they also see their work as essential and important,” she said.
Indeed, CDER approved “many new drug therapies to help a wide range of patients suffering from many different medical conditions to gain new hope for improved quality of life, and in some cases, improved chances of surviving life-threatening illnesses. Many of these new therapies were approved for patients with rare diseases,” spanning a broad spectrum of disease categories, the CDER report states.
Interestingly, the pandemic appears to have helped the agency streamline various operations, leading to what some hope will be permanent changes that drive efficiency and promote collaboration with industry. Among those could be revised agency administrative and procedural changes to expedite and assist with reviews, which clearly would speed up drug development time, said Nancy Bradish Myers, president and CEO of Catalyst Healthcare Consulting Inc., at a Dec. 16 Coalition webinar, “FDA 2021 Outlook: Keeping a Collective Foot on the Medical Product Accelerator.”
Myers mentioned the following innovative programs that were launched during 2020:
- Coronavirus Treatment Acceleration Program (CTAP): initiated by CDER & CBER to increase coordination for moving new treatments to patients rapidly while maintaining a focus on safety;
- COVID-19 Pandemic Recovery and Preparedness Plan (PREPP) to support cross-agency collaboration and communication; and
- Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), a public-private partnership to foster collaborative framework to develop an international strategy for coordinated research response.
Myers added that the new drug approval numbers indicate that the FDA juggled many balls in the air while it was figuring out and implementing these new programs.
Partly as a result of the pandemic, but also due to the workload involved in preparing for FDA advisory committee meetings, the agency also appears to be convening these meetings less frequently, according to a Jan. 8 Pharmalot/Stat+ article, stating that in 2020, only 38 percent of the novel drugs approved went to an advisory committee meeting, compared with 48 percent in 2019 and 51 percent in 2018.
CDER’s 2020 performance regarding drug approvals, as well as the 10-year drug approval trend, is a positive indicator, especially in trying times, according to Bigelow. “The introduction of innovative drugs is important both to the health and well-being of the American public, and also to the healthcare communications industry,” he said.