Feb. 17, 2020 — The Food and Drug Administration (FDA) began 2020 with continued momentum on drug approvals, a significant budget increase and a newly-confirmed Commissioner — Dr. Stephen Hahn – who has not yet presented his priorities for ongoing operations and new initiatives. But President Trump’s fiscal 2021 budget—released Feb. 10 – raises some questions as to whether this momentum will persist.
“The 2021 budget process was well advanced when Dr. Hahn was confirmed, and the president’s budget requests may not fully reflect the Commissioner’s priorities,” noted Jon Bigelow, executive director of the Coalition for Healthcare Communication.
In the budget for fiscal 2020 (which began Oct. 1, 2019) that was agreed upon on Dec. 20, the FDA received an increase of $91 million, or 3 percent, taking the total to $3.16 billion in budget authority funding. Although the increase was smaller than President Trump’s original request of $350 million, in the context of difficult budget negotiations it represented a very good result. Of the increase, $48.9 million is allocated for medical product programs, $31.5 million is allocated for food safety programs, and $12.1 million is allocated for infrastructure improvements across the FDA. Note that these amounts do not count user fee revenues, nor $75 million in funding from the 21st Century Cures Act.
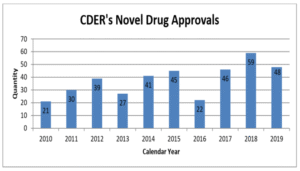
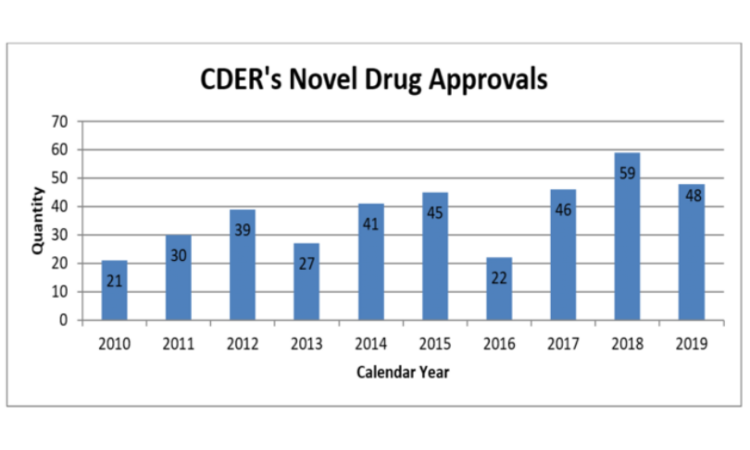
One of the most visible metrics for the FDA – and one of the most important for pharma marketing companies – is the number of new molecular entities approved. In 2019, the Center for Drug Evaluation and Research (CDER) approved 48 novel drugs, just below the recent record of 59 set in 2018. Of the drugs approved last year, 69 percent were approved in this country before any other country, and 60 percent were designated either Fast Track, Breakthrough, Priority Review, or Accelerated Approval. CDER also reports that it met 100 percent of PDUFA user fee dates in 2019.
The number of approvals fluctuates from year to year, but it has been rising over time: the FDA approved an average of 44 NMEs per year from 2015-2019, compared to 32 from 2010-2014, and 21 from 2005-2009.
President Trump’s budget for fiscal 2021 proposes another increase for the FDA, but it is smaller than in recent years: $47 million (1.5 percent), for a total of about $3.21 billion. Even this could be considered a victory, because two general themes in the overall budget are significant increases in military and border security spending and deep cuts in non-defense spending. The 2021 budget proposes major cuts in spending for Medicaid, as well as decreases at some health-related agencies; for example, the budget calls for a 9-percent cut in funding for the Centers for Disease Control and a 7-percent decrease in funding at the National Institutes of Health.
The 2021 budget includes another significant recommendation: that responsibility for regulating the tobacco and vaping industry be removed from the FDA. The budget document says this will “allow the FDA Commissioner to focus on its traditional mission of ensuring the safety of the nation’s food and medical products supply,” but opponents of the tobacco industry charge the Trump administration is trying to weaken regulation of vaping.
“We hope the smaller budget request and the removal of tobacco regulation from the FDA’s purview does not point to lessened support for the important work done by the FDA,” Bigelow said.
CDER Annual Report for 2019: Novel Drug Approvals